Manufacturing & Facilities
Manufacturing
Precision in Every Dose,
Excellence in Every Batch
At Global Pharma, manufacturing excellence begins with our state-of-the-art facility in Alathur, Chennai, strategically located in a government-supported pharmaceutical hub. Purpose-built to deliver precision and consistency, our plant produces a wide spectrum of medicines, including tablets, capsules, injectables, eye drops, ointments, gels, creams, liquid orals, sachets, and nutraceuticals
Our operations are backed by international certifications such as European GMP approval, WHO-GMP and multiple ISO standards (ISO 9001:2008, ISO 9001:2015, ISO 14001:2015, OHSAS 18001:2007, ISO 22000:2005, ISO 17025:2005) , ensuring every batch meets the highest global benchmarks for safety and quality. We are proud to combine innovation and reliability, with a vision to scale, sustain, and serve healthcare needs worldwide.
Built to Power Partnerships, Globally
Global Pharma's advanced facility in Alathur, near Chennai, India, is more than a production site, it's a partner platform. Purpose-built for scale, flexibility, and compliance, our operations give you the confidence to bring products to market faster, with reliable supply and uncompromising quality.

Core Capabilities
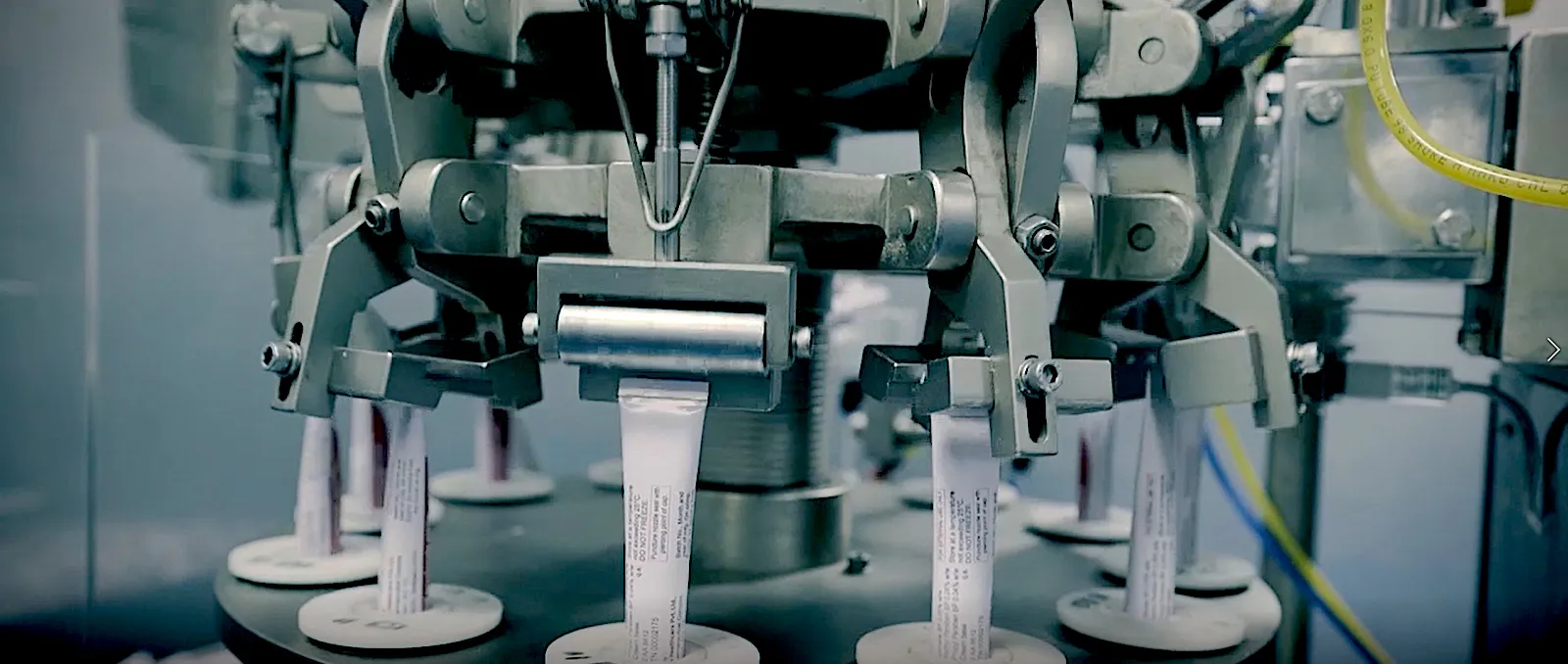
75,000+ sq. ft. facility designed for tablets, capsules, ointments, orals, and injectables. Continuously expanding.
Cleanrooms from Class 100 to Class 100,000 ensure strict environmental control.

Regulatory Confidence
Approved in 40+ countries with 20+ Ministry of Health (MoH) site visit audits.
Certified for WHO-GMP; ISO 9001:2015; ISO 14001:2015; OHSAS 18001:2007; ISO 22000:2005; ISO 17025:2005.
Dedicated compliance team ensures readiness for stringent regulatory authority (SRA) audits.
Supply Chain & Reliability
Robust Packaging
Advanced packaging lines (Alu-Alu, blister, strip packs) ensure product stability and patient safety.
On-site Warehousing
Dedicated warehousing with secure, segregated storage for sensitive APIs, ensuring compliance and efficiency.
Multi-source Strategy
Diversified sourcing of raw materials guarantees uninterrupted supply and resilience against disruptions.
Global Delivery
Proven track record of reliable, on-time delivery to partners and markets worldwide.
Partner Advantages
Speed to Market
High-capacity lines reduce lead times.
Scalable Flexibility
Capable of handling both high-volume generics and niche specialty products.
Risk Mitigation
Redundant utilities (power, water, HVAC) protect production continuity.
Collaborative Approach
Technical transfer support and dedicated partner teams.
Future-Ready Investments
Automation
Automation and digital monitoring to improve consistency and reduce downtime.
Sustainability
Sustainability initiatives: water recycling, energy-efficient HVAC, and waste reduction.
Continuous Upgrades
Continuous upgrades aligned with emerging regulatory and market demands.
Contract Manufacturing
Global Pharma provides fully integrated development and manufacturing solutions. We have an independent manufacturing line ready to fulfill customer needs. Our offering consists of a full range of services, including preformulation, formulation, development, process characterization, scaling up, process validation, and commercial supply. Each stage is supported by state of the art analytical labs and advanced techniques such as Quality by Design (QbD), experimental design (DoE), etc are applied as per protocol customer requirements. Our qualified team of scientists, supported by rigorous project management, will ensure that your project is handled expertly and delivered in a timely and cost efficient manner.






